Article Text
Abstract
Introduction Patients’ failure to adhere on tuberculosis (TB) treatment leads to drug resistance, relapse and death. Non-adherence to TB treatment is higher during continuation treatment phase. The study aimed to evaluate effectiveness of combined pill refilling and medication reminders on adherence to TB treatment.
Methods and analysis A two-arm randomised controlled trial on adult patients with TB was used during continuation treatment phase. In the first arm, in addition to usual care, participants will receive cellphone-based daily medication and weekly pill refilling reminders. In the control arm, participants will receive only usual care. The study will use a covariate adaptive randomisation technique to balance covariates during allocation. The primary outcome is patients’ adherence to TB treatment and secondary outcomes are attendance to clinic and treatment outcomes. We apply intention to treat with generalised linear mixed model.
Ethics and dissemination Ethical approval was obtained from Institutional Review Board of University of Gondar. Written informed consent was applied during enrolment. We will publish findings in peer-reviewed, scientific journals and conferences.
Trial registration number PACTR201901552202539.
- short message service
- patient appointment
- treatment adherence
- treatment outcome
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Non-adherence to long-term therapies are associated with poor health outcomes and increased healthcare costs.1 Patients’ failure to complete tuberculosis (TB) treatment results in drug resistance, relapse and death.2 3 The ability of patients to optimally follow long-term treatment plans is often determined by an interplay of five sets of factors: healthcare team and system related, socioeconomic, condition related, therapy related and patient related.1 4 Non-adherence to TB treatment has been a major challenge in Ethiopia.5–8 In Ethiopia, poor provider–patient relationship and communication, poor knowledge towards TB and its treatment, distance to the health facility, competing employment and family commitments and adverse clinical experiences9 10 were most commonly reported reasons for non-adherence to TB treatment. Forgetfulness accounts to 34% among all reasons for non-adherence.5 11
During continuation TB treatment phase, patients take their daily pills at home with the intention of under the follow-up of treatment supporters. Unlike intensive phase, all daily medication is home based and weekly refill their drugs from the clinics during continuation phase.12 Consequently, non-adherence to treatment was more frequent during continuation phase.11 12
Mobile technologies were found useful for resource-limited countries to overcome barriers against access to healthcare and the quality of care delivery.13 Studies have shown that cellphone reminder systems were feasible and acceptable to improve TB treatment.14 15 Mobile cellular penetration in Africa has reached 66% and estimated to grow stronger than ever and in other regions of the world.16 Currently, mobile subscriptions have reached up to 40 million in Ethiopia.17 A study in Northwestern Ethiopia also showed that 76% of patients on antiretroviral therapy (ART) owned a cellphone, and 51% of them were willing to receive medication reminders.18 Randomised controlled trial (RCT) studies in Pakistan19 and in China20 have shown that daily short message service had no significant effect on patient adherence on TB treatment. Both studies focused on medication reminder but did not address pill refilling reminder; however, both pill refilling and home medication were inseparable. This study will apply a combined intervention package of daily medication and weekly appointment reminders during continuation phase to improve adherence towards TB treatment. The study applies graphic and text reminders to engage illiterate patients. This study aimed to evaluate effectiveness of cellphone-based medication and pill refilling reminders on patients’ adherence to TB medications.
Methods
Study design
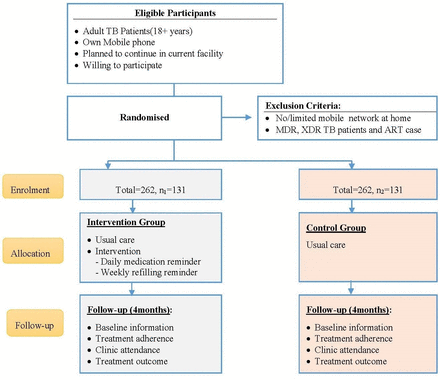
We under take a two-arm, multicentre RCT at selected health facilities in Northwest Ethiopia. The study population includes all adult (>18 years) patients with TB enrolled in continuation phase of TB treatment. Patients enrolled in intensive phase of TB treatment; aged >18 years; own a cellphone; decided not to transfer to another facility and able and willing to provide informed consent are eligible for enrolment. The study excludes patients who started continuation treatment phase; no/limited mobile networks at participant’s home; with multidrug-resistant TB; enrolled to ART and already enrolled in another study that affect outcome of interest.
Intervention package
Participants in the first arm, in addition to usual care, will receive daily medication and weekly pill refilling reminders using graphic and short text messages with local language (Amharic). The graphic reminder intended to engage illiterate is intended to engage illiterate patients who cannot read text messages. Graphic message on figure 1A represents pills for daily medication. The number of daily pills to ranges from two to four based on their body weight. A strip of pills represented on figure 1B aimed to remind clinic appointment for pill refilling as shown in figure 1.
Graphic and Amharic text message to remind weekly pill refilling and daily medication, Northwest Ethiopia, 2019.
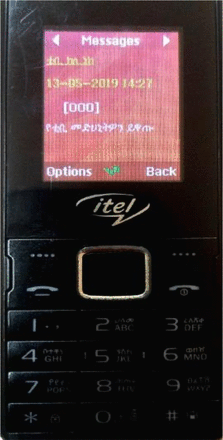
The Amharic message for daily pill reminder ‘እባክዎ የቲቢ መድሀኒትዎን ይዋጡ’ means ‘Please take your pills’. The weekly based Amharic message ‘የክሊኒክ ቀጠሮዎ ነገ ስለሆነ እባክዎ በሰዓቱ ይገኙ!’ means ‘your clinic appointment is on tomorrow, please come on time’. The combined graphic and text messages are displayed and compatible for both smart and simple cellphones, as shown in figure 2.
Cellphone compatibility for graphic and text messaging with Amharic language, Northwest Ethiopia, 2019.
Daily medication reminder is sent on 07:30–08:00 every day to remind the conventional time (08:30) for taking daily TB pills, whereas pill refilling reminder is sent a day before the due date (appointment date) at 18:30–19:00. Before initiating the intervention, each participant on intervention arm will receive a 5 min orientation on use of graphic and text messages. We will pilot the system involving participants out of the main study area.
Usual care
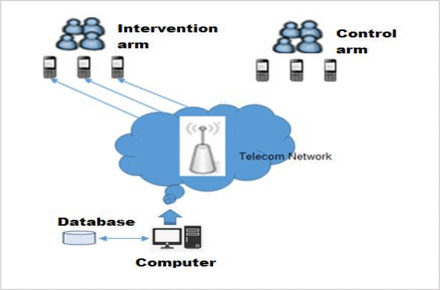
The control arm will receive only usual care. In this study, patients taking their daily medication at home with support of TB treatment supporters (TTS) during continuation phase is considered as usual care. TTS is either health extension worker, family member, neighbour, workmates or community figures and trained to observe the optimal administration TB treatment outside the health facility.21 This study compares treatment adherence between intervention and control groups (see figure 3).
Weekly pill refilling and daily TB medication reminder system, Northwest Ethiopia, 2019.
The study will be conducted from 15 April to 15 September 2019, an average of 2 months for open cohort enrolment of participants and 4 months (122 daily doses) for follow-up period.
Outcomes
Adherence to TB treatment as primary outcome is assessed with self-report using Adherence to Refills and Medications Scale.22 As secondary outcome, pill refill visits measured using at least once for a medication refill visit were classified as non-adherent.23 The study will also measure treatment success rate, defined as the sum of patients cured and/or completed their treatment.24
Sample size determination and sampling procedure
We calculated the sample size using STATA V.14 considering superiority design and the proportion of patient adherence to treatment during continuation phase (π1=66%) in non-intervention group and π2=81% in intervention group.8 We considered 15% minimum detectable effect size, with α=5%, power (β) of 80% and attrition rate of 10%, the total sample size was 262 (131 in each study arms). It is a multicentred study conducted in 13 districts and 1 town administration. Overall, 19 health centres, one health centre from each district and subcity, were included in the trial.
Eligibility criteria
The study recruits participants who fulfil the criteria: age 18+ years, own a mobile phone, planned to continue in current facility and willing to participate in the study, whereas those who are physically impaired (unable to see) will be excluded.
Recruitment
In communication with focal person of the clinic, the research assistant will take the list of all patients with TB enrolled in intensive phase. The research assistant will keep a record for all patients enrolled in intensive phase irrespective of eligibility criteria. After checking eligibility criteria, the research assistants will explain details of the study and fill consent form including reasons for rejection to participate in this trial. The procedure continues in open cohort until required sample size is captured (see figure 4).
Flow of RCT implementation on cellphone reminder intervention to support TB treatment, Northwest Ethiopia, 2019. MDR, multidrug resistant; RCT, randomised controlled trial; TB, tuberculosis.
Randomisation and allocation
The research assistants will be responsible for randomisation and enrolment of study participants. The study will use a covariate adaptive randomisation technique to control and balance the influence of covariates on the study outcome.25 Participants’ educational status (literate and illiterate) and type of facility (health centre and hospital) were considered during randomisation to account individual and service related effect on the outcome variable. We randomly include one patient if more than one patient attending TB treatment from one household.
Masking
We cannot mask study participants due to the nature of the intervention that requires an overt participation in the study. However, outcome assessors and investigators will be masked.
Follow-up
The follow-up period will be 4 months beginning from the time when the patient enrolled in continuation treatment phase. Participants will receive 138 messages (122 daily medication reminders and 16 weekly pill refilling reminders). Data collectors will follow and capture end line data on patient adherence through patient self-report on third month of continuation phase and attendance to appointments and treatment outcomes using register review on fourth month of continuation phase.
Statistical methods
Data analyst will use summary measures like mean, median, SD and percentages. The study will apply intension-to-treat analysis technique. We will use t-tests or Kruskal-Wallis test based on statistical distribution to compare the mean adherence score between intervention and control groups. A generalised linear mixed model to take account the lack of independence of the outcomes of repeated measures of adherence and appointments for the same patient. A χ2 test will be used to compare the proportion of participants attended clinic appointments as well as the proportion of participants with successful treatment.
Discussion
A combined intervention package of daily medication and weekly pill refilling reminders during continuation phase was used to improve adherence towards TB treatment. It has minimal risk and no vulnerable participants associated with the study intervention. However, message fatigue and accidental status disclosure directly associated with the study intervention during informed consent. This reminder system is not to replace the existing services but only to supplement and strengthen the clinical service. The research assistant will obtain written consent from each respondent after explaining the purpose and objectives of the study including the right to refuse or stop at any point in the study without compromising the usual service given by the clinic. The final study report will follow the Consolidated Standards of Reporting Trials checklist. We will publish the study findings in peer-reviewed scientific journals and present in workshops and research conferences.
The finding of the study will provide insight to develop interventions to enhance patient adherence to TB treatment.
Acknowledgments
Authors would like to acknowledge University of Gondar for their approval and facilitation. They are also grateful to Amhara Public Health Institute (APHI), Zonal and District health administration for their willingness to conduct the study.
.
Footnotes
Contributors KDG and BT were involved on scheming research questions; all authors participated on selecting appropriate research design and approved the protocol.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.