Article Text
Abstract
Introduction Different countries use a variety of methods to manage the newborn screening data. In this study, we aimed to compare the experiences of the selected countries to propose a framework for managing the newborn screening data in Iran.
Methods In this comparative study, data were collected using electronic databases and the official website of the Department of Health in America, England and Australia. Data related to the process of newborn screening in Iran were collected using an open-ended questionnaire and reviewing the published documents.
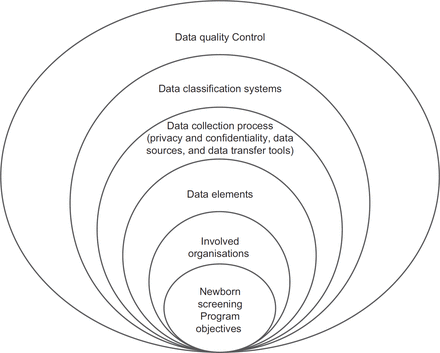
Results In this study, a framework for newborn screening data management was proposed which consisted of six main areas, namely; objectives, involved organisations, data elements, data collection processes, data classification systems and the methods of controlling data quality.
Conclusion The framework suggested in this study can help to re-organise the process of newborn screening with more focus on data management. These data can be used in conducting research and setting strategies for improving the quality of child health in the country.
- neonatal screening
- newborn
- health information management
- public health
Commons license http://creativecommons.org/licenses/by/4.0/
Statistics from Altmetric.com
INTRODUCTION
One of the main national investments in each country is monitoring the health status of newborns and children in the community. Since children are at risk of various diseases, such an investment may largely improve their health status and as a result, the community will move towards a healthier future.1,2 The newborn screening is one of the approaches that have prevented a number of diseases and congenital disorders in the past 40 years.3,4 This approach aims to identify a disease or a disorder before causing irreparable damages. For example, genetic disease screening empowers public health in identifying inherited diseases and helps to prevent or cure such diseases.5 The newborn screening programmes have been started in many developed countries since nearly three decades ago and today, they are part of the newborn health care initiatives. Despite the importance of the newborn screening programme, the developing countries have faced a number of challenges, such as medical, technical and logistical support when implementing the programme and as a result, it has not been conducted in all areas.6,7
In Iran, the centre of endocrine and metabolism research has initially implemented the screening programme for congenital hypothyroidism in 1998.8 Overtime, the national newborn screening programme was developed and now it includes screening for congenital hypothyroidism, phenylketonuria and glucose-6-phosphate dehydrogenase deficiency. However, the main problem can be seen after diagnosis because there is no long-term follow-up of children who have been diagnosed with hypothyroidism, particularly when their health report shows a normal growth pattern. Moreover, some parents refuse to track the health status of their children after diagnosis or refuse to receive further treatment after getting a normal laboratory result.9
In Iran, like many other countries, there is a healthcare referral system composed of primary, secondary and tertiary care. At the primary care level, the health centres at the urban and rural areas are the most important organisations that have a key role in improving health status and providing healthcare services. Regarding the newborn screening, in addition to hospitals, the selected centres are responsible for conducting laboratory tests, collecting data and sending them to the specialists in a timely manner. However, there are different methods of data collection and reporting which have caused unavailability of needed data at the right time and right place, as well as increasing the healthcare costs.10 For example, parents and paediatricians should be informed about the abnormal newborn screening results as early as possible. However, there might be a delay in confirming the lab results or a delay in contacting the families and physicians.11 Therefore, to make screening data available to the healthcare providers, these data should be managed in a structured manner. Otherwise, the improper management of data and a lack of timely follow-up may cause irreversible damages for the patients and public health. In Iran, all data related to the newborn screening are paper-based and reported to the department of health of medical universities. As the country is moving towards using electronic health records and a well-structured paper-based record can be a basis for the future electronic health records, this study aimed to compare the experiences of the selected countries (America, England and Australia) to propose a framework for managing newborn screening data in Iran. It is expected that defining a framework for recording, storing and sharing information helps to increase efficiency and improve health management through informed decision making.
METHODS
This was a mixed methods study which was conducted in 2014. Initially, the data management methods for the newborn screening were compared in America, England and Australia. Data related to the newborn screening systems in America, England and Australia were collected through searching databases, such as PubMed, Science Direct, Scopus, Web of Knowledge and the official websites of the Ministry of Health of these countries. The keywords used for searching databases included neonatal screening, newborn screening, newborn screening information system and screening information system.
Since there was not any specific system for managing newborn screening data in Iran, the data were collected by distributing questionnaires (eight open-ended questions) among the experts in the field of newborn screening (n = 20) to know about the process of newborn screening and the methods of data management. These data were analysed using content analysis method. Finally, a framework was proposed based on the results achieved from the document review and experts’ opinions.
RESULTS
As noted before, there was no adequate document about the newborn screening data management in Iran. Therefore, a questionnaire consisted of eight open-ended questions was distributed among 20 participants who worked in the field of newborn screening. Table 1 shows the demographic characteristics of the participants.
Having reviewed the related literature in the selected countries and participants’ opinions in Iran, the main components for managing newborn screening data were determined to propose a framework. These components included the objectives, involved organisations, data elements, data collection process, disease classification systems and the methods of data quality control. The details of each component are presented as follows:
Objectives
The findings showed that the objectives of newborn screening programmes were similar in America, England and Australia. These objectives included disease prevention, information-based planning, investigating the causes of infants mortality, creating new methods for screening, timely follow-up visits, providing a basis for education and research.12–14 Similarly, in Iran, the objectives of the newborn screening programme were patient follow-up, education, information-based planning and disease prevention.15 Obviously, to achieve these objectives, accurate and precise data should be collected and managed as valuable information recourse.
Involved organisations
In Australia, the Genetic Association and the Ministry of Health were responsible for managing the newborn screening data, and the data were collected in each state separately.16 However, in America and England, the newborn screening data were collected at the national level.17,18 In America, a number of organisations, such as the Advisory Committee on Heritable Disorders in Newborns and Children and National Newborn Screening and Genetics Resource Center were involved in the process of newborn screening.17 In England, Primary Care Trusts, UK Newborn Screening Programme Centre, UK National Screening Committee and Public Health England were among the organisations that are involved in the process of newborn screening. In Iran, the newborn screening data related to hypothyroidism disease were collected at the national level and the Ministry of Health was the only organisation responsible for implementing newborn screening programmes.19
Data elements
According to the findings, different data elements were collected during the newborn screening. The data related to a newborn (gender, birth weight, birth time and birth order), a mother (name, date of birth and gestational age), screening tests (the name of the laboratory, the date of sampling and test results), service provider centre (the name of the primary care centre, place of birth and name of the hospital) and service providers in screening centres (the name of the doctors) were collected and recorded in newborn screening cards in America, England and Australia.20–24 In England and Australia, the parents’ consent form was attached to the blood sample card.22,23 Only in Australia, a separate consent form was completed.23 Generally, a comparison between the number of newborn screening data elements in the above-mentioned three countries showed that in England, the number of collected data elements was more than in other countries. In Iran, the demographic information of a newborn, date of sampling, the name of the primary care and the sampling centre, newborn’s father’s name, the number of Guthrie paper, screening test results and the date of test results are recorded.25
Data collection process
The results showed that the process of collecting and recording newborn screening data in electronic patient records were similar in America, England and Australia.22–24 In America and Australia, the newborn screening data were kept in laboratories.23,24 In Australia, there was a central database to store the test results.23 In England, electronic systems and data collection forms were used for collecting newborn screening data.22 In Iran, the newborn screening forms were used to collect data and were kept in screening centres and laboratories. However, there was no electronic system for collecting newborn screening data.25 Having reviewed the literature, it was revealed that three main areas related to the process of data collection were privacy and confidentiality, data sources and data transfer tools which are discussed below separately.
Privacy and confidentiality
According to the literature review, setting privacy and confidentiality laws and obtaining parents’ informed consent were the most common ethical principles in all three countries.26–28 However, in Australia, privacy and confidentiality policies were more complicated to increase the privacy of newborn screening data. These policies included authorising staff to access screening card and to use the electronic system.28 In Iran, the document review showed that there was no specific privacy and confidentiality plan for the newborn screening data.
Data sources
The findings showed that the health/primary care centres, laboratories and the Ministry of Health/National Health Services were the main data sources of newborn screening data in all three countries.18,24,29 In America and England, the obstetric and public health centres were other data sources.18,24 In England and Australia, data were also generated in hospitals to be included in the newborn screening system.18,29 The results showed that compared to America and England, Australia used fewer data sources in the newborn screening process. In Iran, the data sources of the newborn screening system were health centres, laboratories, hospitals and the Ministry of Health.25
Data transfer tools
According to the findings, in America, the newborn screening system used more tools to collect relevant data.30 Moreover, all three countries used email and electronic health records (mother and baby) to collect data.22,23,31 The web-based electronic system was used only in America.12,32 In Iran, however, only phone call and data collection forms were used to transfer newborn screening data and there was no electronic system to collect data.
Data classification systems
The findings showed that newborn screening data were classified using textbooks and various systems in America,33 Australia14 and England.34 In Iran, there was no specific classification and nomenclature system used for newborn screening data management (Table 2).
Methods of data quality control
The findings showed that in America, assigning a unique serial number to the birth certificate and newborn screening card and controlling the serial numbers were among the methods used to control data quality.35 In England, national numbers were used in newborn screening cards along with the barcode technology, numbered tags, standard codes for exchanging newborn screening data, data dictionary, data standards and unique ID numbers in laboratory processes.18 In Australia, the main focus was on the development of data dictionary and standards.36 However, in Iran, there is no mechanism for controlling the quality of newborn screening data.
DISCUSSION
The newborn screening programme is considered a preventive public health programme in many countries. It provides timely information and services to prevent many consequences, such as disability, death and other complications of a newborn’s diseases. While in many newborn screening programmes, less attention has been paid in collecting the related data,37 improving the quality of the programme is dependent on the systematic data collection of screening services.13 The findings of the current study showed that in America, Australia and England, newborn screening data are managed in a systematic manner. In these countries, in addition to the manual systems, electronic systems were used to manage newborn screening data. In Iran; however, only the important data elements, such as the newborn’s demographic information, date of sampling, information about the primary care and the sampling centre, Guthrie paper number and the test results were recorded in paper-based records.38
Having compared the status of the newborn screening data management in three selected countries, a framework was proposed for managing the newborn screening data in Iran. The framework included six main components: objectives, involved organisations, data elements, data collection process, disease classification and the methods of data quality control (Figure 1).
Concerning the objectives of the newborn screening programmes, the findings showed that disease prevention, information-based planning, controlling the quality of the programme and educating healthcare professionals were common in the selected countries. The results are consistent with the findings reported by Olivieri39 who showed that the objectives of a national registry system for hypothyroidism in children were evaluating newborn screening programmes and conducting research and epidemiological studies. Also, Pitt stated that the secondary objectives of using newborn screening cards are quality assurance, detecting other diseases and conducting research.40
Regarding the involved organisations, in Australia, the Genetic Association and the Ministry of Health were responsible for managing the newborn screening data. In this country, the newborn screening data were collected separately in each state41; however, in America and England, the newborn screening data were collected at the national level.32,34 In Iran, the screening data of newborns’ hypothyroidism disease is collected at the national level and other data are collected locally to be reported to the Ministry of Health.25 Moreover, regarding the number of involved organisations in the process of collecting newborn screening data, in America, more organisations were involved in the process compared to England and Australia. These organisations included the screening committee, genetic committee, children with hereditary disorders committee, laboratories community, information management association and centre for health information technology. The results are in line with the statement of American Academy of Pediatrics, in which they noted that providing the best newborn screening services depends on the interaction between the hospitals, healthcare centres and public health organisations.42 Hinman et al.17 reported that the Ministry of Health, laboratories, hospitals, health houses, legislator organisations and insurance companies should be involved in the process of newborn screening. In another study, Livingston et al.43 noted that data obtained from tracking newborn clinical genetic services are important for improving newborn screening programmes, and cooperation between genetic services and organisations which are responsible for implementing newborn screening programmes is necessary. However, In Iran, the Ministry of Health is the only organisation responsible for implementing newborn screening programmes.25 Therefore, it seems that organisational involvement in collecting the newborn screening data may help to improve health management and decision making.
Data collection process is another important component of a newborn screening process. In England, the data collection process started at the birth time by taking the parents’ consent form and taking the baby’s blood sample at the hospital or midwifery services. In this process, the department of newborn health records, primary care centres, laboratories and specialised groups work together to collect related data. Similarly, Hinman et al.17 suggested that the newborn’s family, health centres, clinical care coordinators, support services, laboratories and hospitals need to be involved in the process of screening and data collection. In another study, Padilla et al. stated that the current health records in the public health sector can be used as a ready mechanism for recording the newborn screening data. For example, in the Philippines, the newborn screening results are recorded in the newborn health record; so that, service providers use the results in their preliminary examinations and make decision.6
While collecting the newborn screening data, it is important to consider data privacy and confidentiality issues. The literature review showed that all three countries paid special attention to this issue. According to Botkin et al. taking informed consent from parents is necessary before recording a sick newborn’s data in the national registry of newborn screening. The researchers believed that parents have the right to decide about participating in future research.44 In Iran; however, there is no specific plan to assure the privacy and confidentiality of newborn screening data. As a result, it is necessary to identify different approaches taken by other countries to be able to learn and overcome this challenge.
Regarding data sources, Botkin et al. noted that a national registry for newborn screening data, newborn’s family, healthcare providers at different levels, healthcare centres/health houses and schools can be considered data sources. They believed that a large amount of newborn screening data is collected by speaking with parents. They also indicated that web-based newborn screening systems are appropriate tools for collecting the data.44 However, in Iran, the number of data sources is limited to the health centres, laboratories, the department of health located in the medical universities and the Ministry of Health. Obviously, by paying more attention to the newborn screening programme and its related data, data sources will be identified to be able to collect more accurate and complete data.
In newborn screening programme, not only data sources are important but also data sharing and data transfer are of high importance to be able to manage diseases in a timely manner. Padilla et al. stated that communication tools play an important role in tracking sick newborns, especially when getting access to families is difficult. They introduced smartphones as an appropriate way to educate families to follow newborn screening programmes.6 In another study, Therrell et al. showed that the newborn screening data, birth records and other documents related to public health are integral parts of every person’s electronic health record. Therefore, a unique ID number of newborn screening samples, like Guthrie number, can be used to link patient’s data.35 It is notable that using electronic systems is suggested for managing newborn screening data because it can support different components of the programme, reduce errors and improve effectiveness.6 However, where the time is crucial, the telephone is still used as the first communication tool in many countries. In Iran, data transfer tools were the telephone and paper-based records. Therefore, more attention should be paid and to move forward to use information technology and improve the efficiency and effectiveness of such programmes.
An integral part of quantitative studies and a basis for scientific conclusions is classification.45 In healthcare, the necessity of using classification and nomenclature systems, such as systematic naming of clinical medical terms (SNOMED CT), logical observation codes and identifiers (LOINC), ICD-10-CM, ICD-9-CM and enzyme codes for providing standard laboratory reports and exchanging data in electronic systems, such as electronic health records have been suggested.24 These systems play an important role in organising data and applying obtained knowledge in planning and decision making. Moreover, the interaction between different electronic systems depends upon the application of data classification and standards. In this regard, Goodwin et al.46 suggested that the use of standard terminology and coding systems empowers researchers, clinicians and public health systems to exchange newborn screening data in various states in America. In Iran; however, there was no specific classification and nomenclature system for managing the newborn screening data. Therefore, the use of different types of classification and nomenclature standards, such as LOINC, and SNOMED CT is suggested to be able to analyse and exchange data.
According to the results, in order to control data quality, using the national number in the newborn’s screening card, barcode technology, numbered tags, standard codes for exchanging newborn’s screening data and developing data dictionary, standards and unique ID numbers for laboratory results were suggested.34–36 The results of the 26th session of the Advisory Committee on Heritable Disorders in Newborns and Children in Washington showed that using a serial number on the birth certificate can improve the quality of data in the newborn’s screening process. Moreover, the use of the unique ID number for the newborn’s screening sample can help to link data and health records and facilities patient tracking.14 However, in Iran, there was no specific mechanism for controlling the quality of newborn’s screening data.
While the framework suggested in the current study includes the main components for managing newborn screening data, to be able to collect useful data, a collaboration between different organisations and sources of information is required. To achieve this, effective communication strategies need to be set among the public health, primary care and referral/specialty services to be assured about the continuity and the accessibility of information at the point of need.
LIMITATIONS
Although in this study, a framework was proposed to manage newborn screening data in Iran, the study had some limitations. First of all, due to the time and resources restrictions, only three countries were selected to identify and compare the necessary components of newborn screening data management. Although, these countries had a long-term experience in the newborn screening programmes and had moved towards using electronic systems, including more countries or comparing the methods of newborn screening data management in the developing and developed countries may give a more complete picture of the experiences gained by different countries. The second limitation was related to approving the framework by the experts. The framework proposed in the current study was formed based on the literature review. Further research has been planned to gain the consensus of experts about the appropriateness of the framework and its components.
CONCLUSION
The newborn screening programme is an integrated part of a public health plan which aims to track the status of the newborn patients and to connect healthcare providers, families and related healthcare organisations. In order to manage the newborn screening data in Iran, a framework was proposed based on the experiences of three selected countries. The experiences of the selected countries showed that newborn screening activities should be conducted in a structured manner. As a result, the framework had six main components; namely, objectives, involved organisations, data elements, data collection process, disease classification and the methods of data quality control. The framework suggested in the current study can help to re-organise the process of newborn screening with a focus on managing related data. These data can be used in conducting research and setting strategies for improving the quality of child health in the country.
Acknowledgements
This work was supported by the Iran University of Medical Sciences under Grant 341.
References
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.